Chimeric antigen receptor (CAR) T-cell therapy has transformed blood cancer treatment, but access remains limited due to staggering costs.
At the 2024 Advanced Therapies Week meeting in Miami, Dr. Marcos De Lima outlined his approach to developing cost-efficient CAR-T therapies and delivered his vision for the promise of point-of-care CAR-T cell manufacturing.
Dr. De Lima spearheaded point-of-care CAR-T production first at Case Western Reserve University starting in 2016, and more recently at Ohio State. His teams have shown that on-site bioreactor manufacturing using the same protocols can reliably produce CAR-T cells with robust clinical outcomes comparable to commercial products.
“Half of Europe still lacks access to CAR-T cells simply because they are too expensive,” De Lima stated. At Case Western, his team’s manufacturing costs came to around $27,000 per patient – a fraction of typical $400,000+ price tags. Remarkably, the hospital itself did not lose money due to offsetting inpatient reimbursements.
Through a partnership with Lentigen (now Miltenyi), Marco and his team developed a twist on a familiar CAR construct. The slide below shows the construct with a 4-1BB co-stimulator molecule, the difference is the transmembrane domain that uses a TNF receptor superfamily.
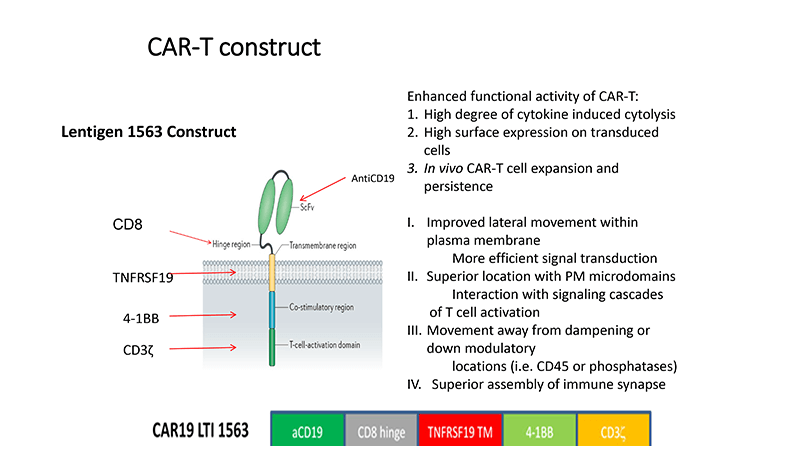
The team went from manufacturing ideas to treating their first patient in under a year, who had high-risk lymphomas and had already received more than two lines of therapy. The process involved an IL-7/IL-15 culture and was done in Miltenyi’s Prodigy platform.
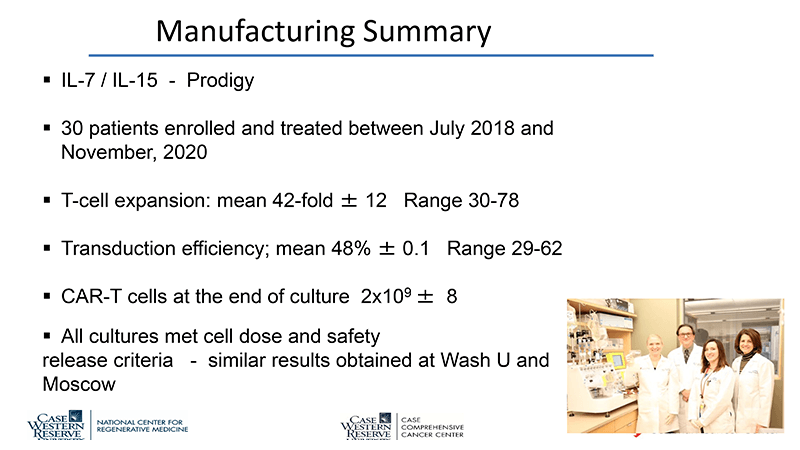
By partnering with a group at UWash and another in Moscow, using the same protocol, vector construct, bioreactor, and culture conditions all in parallel, Dr de Lima was able to generate robust, repetitive, comparable outcomes.
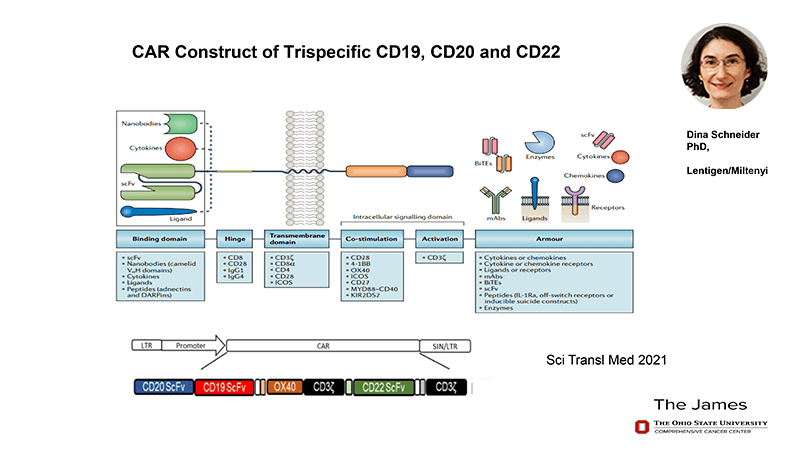
After moving to Ohio State, De Lima continued innovating but with a different vector and working on the hypothesis that by targeting more than one antigen associated with a disease you’re likely to have a better response than by targeting just one. Specifically, his team was working on B cell malignancies that presented CD-19, 20, and 22. In partnership with Dina Schneider, Marco developed a tri-specific CAR construct targeting CD19, CD20, and CD22 and containing an OX40 co-stimulatory molecule. Again, clinical trials were started within a year and manufacturing was reduced to six days.
While hospital-based CAR-T manufacturing could dramatically increase access in the U.S., regulatory acceptance remains a challenge. International initiatives like the Barcelona model distributing from a central facility may pave the way. But how will the advent of allogeneic therapies impact the field? When an off-the-shelf product comes along will it be the end for autologous therapies, or will the cost be magnitudes lower and so render them redundant?
Regardless, De Lima firmly believes hospital-developed and manufactured CAR-Ts will be “doable” in America’s future. He points to organic entrepreneurship stemming from his Case Western program, which spun off three startups. This includes one with a 20-hour overnight, in vivo CAR-T manufacturing process by Kure.AI, which is now in clinical trials and two patients are already in remission.
In its latest endeavor, Ohio State has partnered with the non-profit Caring Cross to transfer point-of-care CAR-T capabilities to Brazil’s public healthcare system. “We’ll be vetting a mobile clean room for manufacturing to be deployed at a facility in Rio de Janeiro,” De Lima announced.
As work continues to globalize affordable CAR-T cell therapy, Marco leaves us pondering a key question: “If you could provide CAR-T for a fraction of the cost, why wouldn’t you?”







